Demaskierung von Endotoxinen | Beitrag aus pharmind 88 | Nr. 1 | Seite 66-70 (2026)
27.02.2026Die Maskierung von Endotoxinen stellt ein Problem bei der Standzeit-Validierung dar, denn sie führt zu falsch-negativen Testergebnissen. Als Auslöser für die Maskierung kommen dabei 3?Mechanismen ...

UDI für Dosierpipetten: MDR-konform, praxisnah und wirtschaftlich umgesetzt
23.02.2026UDI muss nicht kompliziert oder teuer sein. Gerade bei Dosierpipetten der Klasse I zählt eine Lösung, die MDR-Compliance, Wirtschaftlichkeit und Produktionsrealität vereint. elmplastic setzt auf praxisbewährte Direktdruck- und Verpackungskonzepte, die Rückverfolgbarkeit und Patientensicherheit gewährleisten – skalierbar, effizient und marktfähig. So wird UDI vom Kostenfaktor zum echten Mehrwert für Hersteller.
Zwischen GKV-Reform und Standortpolitik: Menschen mit Seltenen Erkrankungen brauchen Rückhalt
27.02.2026Anlässlich des Tages der Seltenen Erkrankungen und im Zuge des laufenden Pharma-Dialogs, der Reformdebatten in der Gesetzlichen Krankenversicherung (GKV) und internationaler Standortdiskussionen – ...
Chemie-Tarifrunde: Verhandlungen erneut vertagt
27.02.2026Die Chemie-Tarifverhandlungen sind auch in der zweiten Bundesrunde ohne Ergebnis geblieben. Die Verhandlungen für 1.700 Betriebe mit 585.000 Beschäftigten werden am 24./25. März in Bad Breisig ...
Unsicherheit im Pharmageschäft
27.02.2026Die deutsche Pharmaindustrie blickt auf ein bewegtes, aber erfolgreiches Jahr zurück. Produktion und Umsatz lagen im Gesamtjahr 2025 deutlich über Vorjahr: Die Produktion stieg um 4,5 Prozent. Die ...
Von nachhaltigen Laboren bis zu neuen Karrierewegen - Zukunftsthemen der Analytik auf der analytica conference 2026
26.02.2026Vom 24. bis 27. März findet die analytica, Weltleitmesse für Labortechnik, Analytik und Biotechnologie, auf dem Münchner Messegelände statt. Begleitet wird sie vom 24. bis 26. März von der ...
Pharma Deutschland: Ethanol-Regulierung für Arzneimittelproduktion steht noch aus
27.02.2026Der Ausschuss für Biozidprodukte (BPC) der Europäischen Chemikalienagentur (ECHA) hat in dieser Woche eine Stellungnahme verabschiedet, mit der er die Zulassung von Ethanol als Wirkstoff in Hand- ...
Tag der seltenen Erkrankungen 2026 – Gesehen werden – gehört werden – verstanden werden
27.02.2026Seltene Erkrankungen sind alles andere als selten. Allein in Deutschland leben rund 4 Millionen Menschen, in Österreich etwa eine halbe Million, mit einer der über 8.000 bekannten seltenen ...
VALIDOGEN stellt synthetische, methanolfreie Promotoren der nächsten Generation für Pichia pastoris vor
27.02.2026Die VALIDOGEN GmbH, ein führendes Auftragsforschungs- und Entwicklungsunternehmen, spezialisiert auf rekombinante Proteinexpression, Stammoptimierung und die Entwicklung von ...
KI-gestützte Plattform von Redwood AI beschleunigt chemische Synthese und optimiert Prozesse entlang des global wachsenden “CDMO”-Marktes
27.02.2026Die pharmazeutische Industrie steht vor einer fundamentalen Transformation. Neue Wirkstoffe werden komplexer, Entwicklungszyklen länger und teurer, und regulatorische Anforderungen steigen ...
Pentaxipharm: FDA erteilt Freigabe für klinische Phase I/II-Studie für CXCR4-basiertes hämatoonkologisches Entwicklungsprogramm
27.02.2026Die Pentixapharm Holding AG, ein biopharmazeutisches Unternehmen mit fortgeschrittenen klinischen Studien mit Fokus auf neuartige Radiopharmazeutika, gibt bekannt, dass die U.S. Food and Drug ...
Durchbruch in der Click-Chemie: Innovative Methode revolutioniert die Arzneimittelentwicklung
27.02.2026Mittlere Moleküle mit einem Molekulargewicht von mehr als 1.000 sind aufgrund der vielen Schritte und des hohen Zeitaufwands schwer zu synthetisieren, was die Entwicklung eines neuen Ansatzes ...
meistgelesen

Beitrag aus der Ausgabe 10/2025 der Zeitschrift pharmind
KI in der Pharmazeutischen Industrie
Hype oder Organisationsaufgabe?
Hype oder Organisationsaufgabe? – Das ist natürlich eine rhetorische Frage. „Hype“ ist negativ konnotiert: Etwas, das rasch wieder vergeht und kaum Erinnerungen hinterlässt. Dies trifft auf Künstliche Intelligenz (KI) allerdings nicht zu. Den Begriff gibt es schon seit beinahe 80 Jahren. In dieser Zeit gab es verschiedene Phasen, die jeweils die öffentliche Diskussion befeuert haben. Es blieb jeweils auch etwas übrig, ...

Beitrag aus der Ausgabe 10/2025 der Zeitschrift pharmind
Betrieb computergestützter Systeme
Sandkühler | Computergestützte Systeme
Die rasante Entwicklung der Informationstechnologie hat dazu geführt, dass computergestützte Systeme in nahezu allen industriellen Bereichen eine zentrale Funktion einnehmen. Insbesondere in der pharmazeutischen und biotechnologischen Industrie sind diese Systeme für die Verwaltung und Verarbeitung sensibler Daten, die Steuerung und Überwachung von Produktionsprozessen sowie für die Einhaltung regulatorischer Vorgaben unverzichtbar. Mit der ...
Beitrag aus der Ausgabe 10/2025 der Zeitschrift pharmind
Pflanzliche Arzneimittel unter Druck
Markt, Regulierung und Zukunftsperspektiven – Teil 1
Pflanzliche Arzneimittel (Phytopharmaka) sind ein wichtiger Bestandteil in unserem Gesundheitssystem und haben sich besonders in der Selbstmedikation etabliert. Sie sind leicht verfügbar und eignen sich v. a. für die Behandlung leichter Beschwerden und Erkrankungen. Am häufigsten werden sie als Mittel gegen Erkältungserkrankungen, Magen- und Verdauungsbeschwerden sowie als Beruhigungsmittel eingesetzt [1]. In der Regel zeichnen sich ...
Top Themen

Beitrag aus der Ausgabe 2/2026 der Zeitschrift pharmind
Reinmedienprojekte in der Pharmaindustrie
Warum die Validierungsdokumentation den entscheidenden Mehraufwand bedeutet
Die GMP-gerechte Planung und Umsetzung von Reinmedienanlagen in der pharmazeutischen Industrie erfordert ein umfassendes Validierungs- und Dokumentationsmanagement, das die regulatorischen Anforderungen von Beginn an berücksichtigt. Systeme zur Erzeugung und Distribution von Gereinigtem Wasser (Purified Water, PW) und Wasser für Injektionszwecke (Water for Injection; WFI) berühren das Produkt direkt und müssen daher sämtliche Spezifikationen ...

Vorschau (Änderungen vorbehalten)
Beitrag aus der nächsten Ausgabe 3/2026 der Zeitschrift pharmind
(erscheint am 31.03.2026)
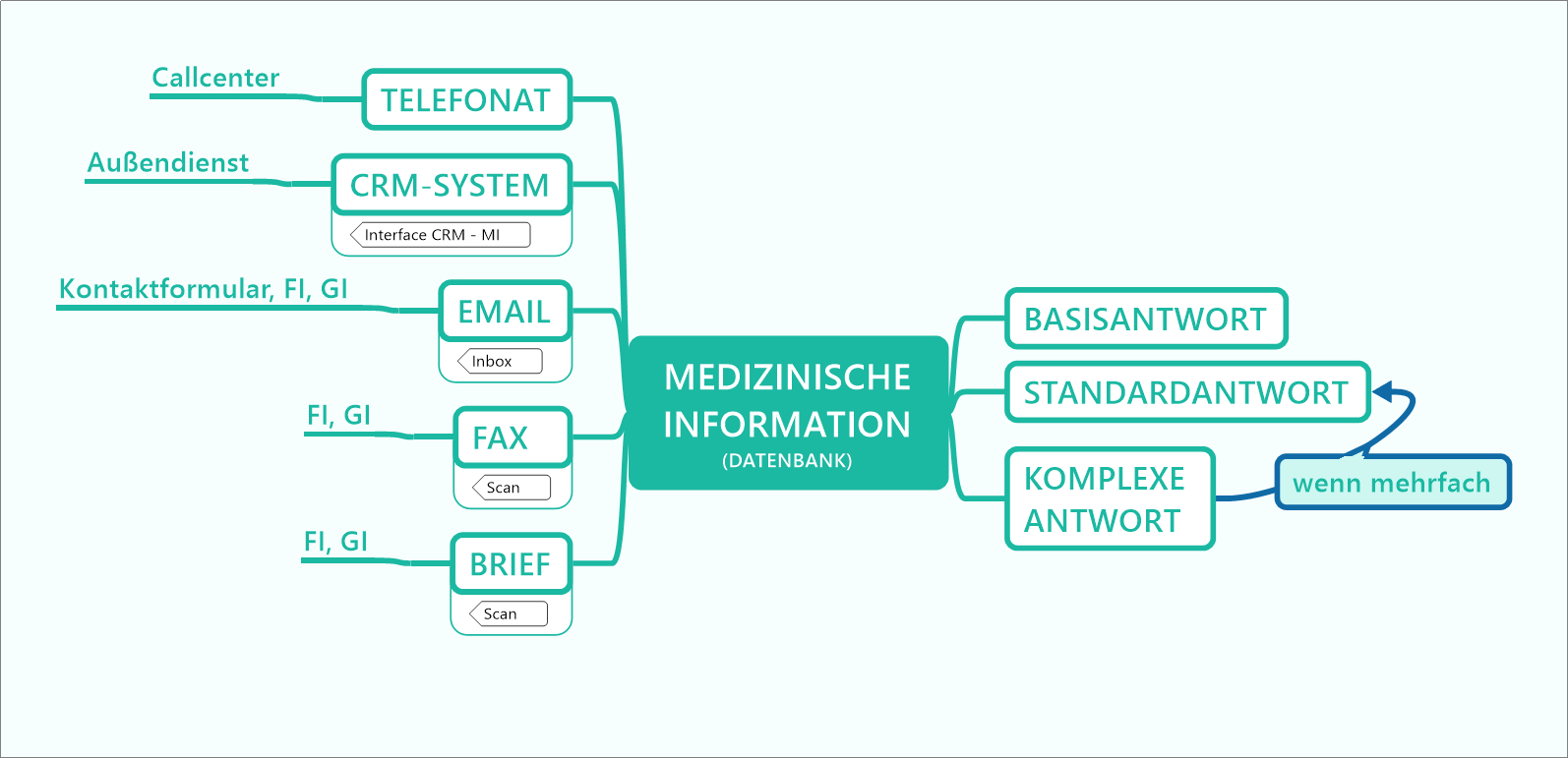
Standard Reference Documents per Online-Recherche | Dokumententyp für den erweiterten Wissenstransfer an Heilberufler
Standard Reference Documents (SRDs) sind ein in vielen Pharmaunternehmen etablierter Dokumententyp, mithilfe dessen vertiefte Anfragen von Heilberuflern beantwortet werden. Dieser Dokumententyp kann nun über die etablierten Kommunikationskanäle wie Telefon, E-Mail, Außendienst oder Post hinaus auch über einen digitalen Kanal angeboten werden. Damit kann eine asynchrone Informationsbeschaffung, also ohne die unmittelbare Weitergabe der Information durch einen Mitarbeiter, umgesetzt werden. Arzneimittelkompendien sind eine ideale Schnittstelle für die digitale Anbindung dieses Dokumententyps als niederschwelliges Instrument für den digitalen Wissenstransfer zwischen Pharmaindustrie und Heilberuflern und dienen damit der Sicherstellung der Arzneimitteltherapiesicherheit.