Comparison of the Performance of Single Punch and Rotary Tablet Presses from Different Vendors
Maschinen- und Anlagenbau
Key WordsTablets | Tensile strength | Rotary tablet presses | Single punch tablet presses | Force-time profiles
Abstract
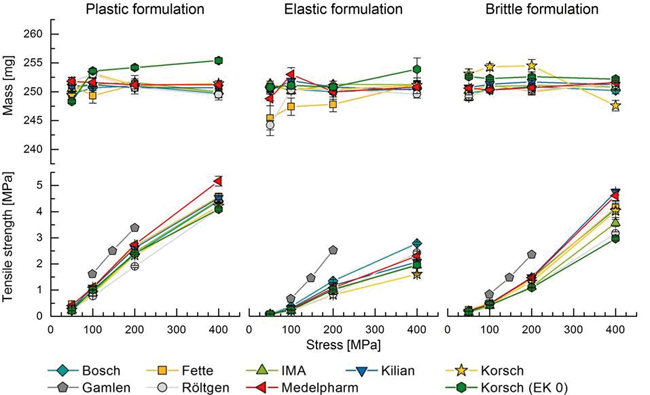
As tablets are the most common dosage form for drug delivery, many different rotary presses are available on the market in order to produce tablets with a high throughput. Single punch presses are used for pharmaceutical development or to simulate the compaction process using small material quantities. In this study, 5 different rotary presses and 4 single punch presses were utilized using 3 different model formulations. Tablet masses and tensile strengths were investigated as a function of compaction force and process time. The corresponding force-time profiles were analyzed as well. In general, significant differences between the presses were observed even if the deviations were rather small from a practical point of view. The lowest variations of tablet properties were found using the plastically deforming formulation, while the elastic formulation was more challenging. High throughput with comparable high tablet tensile strength can be achieved by the brittle formulation. By analyzing representative force-time diagrams it was found that dwell times varied considerably between the different machines.
Annette Bauer-Brandl · Department of Physics, Chemistry and Pharmacy, University of Southern Denmark, Odense (Denmark)
Peter Kleinebudde · Institute of Pharmaceutics and Biopharmaceutics, Heinrich-Heine-University, Düsseldorf (Germany)
Correspondence:
Markus Thommes, Department of Biochemical and Chemical Engineering, TU Dortmund University, Emil-Figge-Str. 68, 44227 Dortmund (Germany); e-mail: markus.thommes@tu-dortmund.de
Abstract
As tablets are the most common dosage form for drug delivery, many different rotary
Sie haben Tech4Pharma / cleanroom & processes für sich entdeckt und möchten auf alle Beiträge und Ausgaben Zugriff haben?
Dann registrieren Sie sich noch heute kostenlos und genießen Sie sofort alle Möglichkeiten – recherchieren, lesen, downloaden.