Influence of X-ray Radiation as PAT Method on the Model Substances Tramadol HCl and Nifedipine Compared to the Influence of UV-Vis Radiation
Messen/Steuern/Regeln
Summary
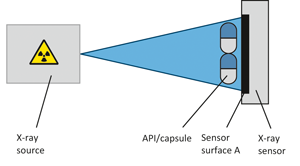
Bosch has recently developed an X-ray technology, which can be implemented in a capsule filling machine (GKF) or as a stand alone visual inspection control unit (KKX), fulfilling PAT purposes. This technology is applicable for 100 % check weighing, detection of foreign particles (e. g. metal), measurement of capsule conditions (length, deformation) and provides information about the filling process.
The measurement principle is based on the Lambert-Beer law. Intensity of the incident X-ray radiation is attenuated dependent on the sample passed – through distance. The absorbance A, the negative logarithm of the quotient of incident intensity and transmitted intensity, is directly proportional to the distance transmitted, which can be related to the mass of the transmitted sample.
Due to the short measurement period of the X-ray technology in the range of 250 ms to 1 s, this technique allows for 100 % inline weight control of capsules and is considered to be a non – destructive technique.
In the present study, the influence of X-ray radiation on sample stability in comparison to environmental radiation sources like daylight was investigated for Nifedipine. This API is known to undergo photodegradation to dehydronifedipine upon exposure to UV light and to the nitroso analogue of dehydronifedipine when exposed to sunlight. Additionally, the influence of radiation to Tramadol HCl was analyzed, which is known to be stable even under accelerated stress conditions in solution.
For both, Nifedipine and Tramadol, no degradation was observed if exposed to X-ray radiation for a period of 2 hours. Tramadol HCl was stable even after exposure to artificial daylight for a period of 30 minutes as expected.
Nifedipine showed increasing photodegradation over time to the main degradation products Nifedipine Impurity A (dehydronifedipine) and Nifefedipine Impurity B (nitroso analogue of dehydronifedipine) as well as to several other unknown degradation products. After 30 min exposure to artificial daylight Nifedipine showed a total degradation of 10.06 % including 0.44 % Nifedipine impurity A, 9.46 % Nifedipine impurity B and 0.16 % unknown degradation products.
Corresponding Author:
Martin Vogt, Robert Bosch GmbH; Packaging Technology, Stuttgarter Str. 130, Waiblingen; e-mail: martin.vogt4@bosch.com
Summary
Bosch has recently developed an X-ray technology, which can be implemented in a capsule filling machine (GKF) or as a stand alone visual inspection control unit (KKX), fulfilling PAT purposes. This technology is applicable for 100 % check weighing, detection of foreign particles (e. g. metal), measurement of
Sie haben Tech4Pharma / cleanroom & processes für sich entdeckt und möchten auf alle Beiträge und Ausgaben Zugriff haben?
Dann registrieren Sie sich noch heute kostenlos und genießen Sie sofort alle Möglichkeiten – recherchieren, lesen, downloaden.