Material selection as a control strategy to limit hydrogen peroxide residuals in drug products
Reinraum
Key WordsIsolator | Diffusion | Hydrogen Peroxide | Decontamination | Material Exposure | Aseptic Processing
Summary
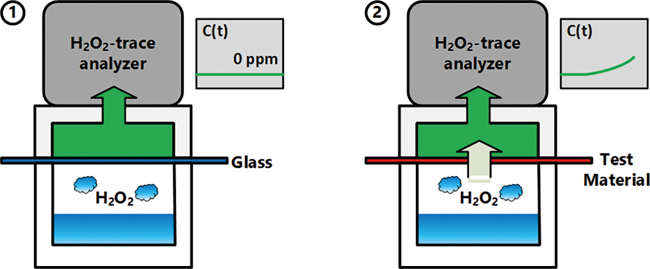
Aseptically processed pharmaceutical products and packages may be exposed to hydrogen peroxide (H2O2) throughout the filling and packaging process. While the advantage of the decontamination with H2O2 lies in its harmless end-products water and oxygen, residual hydrogen peroxide may show detrimental effects on sensitive pharmaceutical products, even at trace concentrations. Therefore, the transmission rate of H2O2 through the packaging material (e.g. polymer packaging foils) during a decontamination process needs to be determined to prevent H2O2 ingress into the product. Thus, in this study, a methodology capable of generating H2O2 interaction data is proposed, which may be used for packaging material selection.
Correspondence:
Davide Ravasio, SKAN AG, Binningerstrasse 116, 4123 Allschwil (Switzerland); e-mail: davide.ravasio@skan.ch
 | Claudia Widmann In 2017 Claudia Widmann joined the research department of SKAN AG. Since then, she was working on different research topics, such as H2O2 sensing, H2O2 ingress into packaging materials and material testing in general (e.g. resistance of materials to H2O2). Today she organizes the execution of scientific customer studies, performed at SKAN AG. |
 | Davide Ravasio In 2019 Davide Ravasio joined SKAN AG as PhD, Research Engineer Chemistry in the Research and |
Sie haben Tech4Pharma / cleanroom & processes für sich entdeckt und möchten auf alle Beiträge und Ausgaben Zugriff haben?
Dann registrieren Sie sich noch heute kostenlos und genießen Sie sofort alle Möglichkeiten – recherchieren, lesen, downloaden.