A Case Study to Introduce Process Analytical Technologies in Pharmaceutical Manufacturing
Messen/Steuern/Regeln
Key Words OSD Secondary Manufacturing | PAT | Quality by Design | Spectroscopy | Data Processing | MVA
Abstract
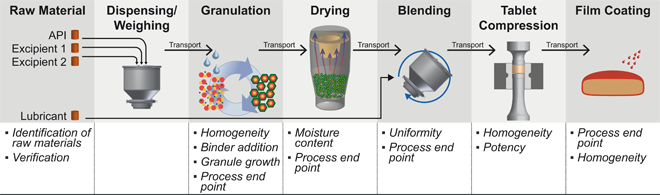
Today, Process Analytical Technologies (PAT) are well recognized as a set of tools within the pharmaceutical industry. However, the application for in-line or on-line measurements did not find the same response throughout the industry. Instead, the level of implementation ranges from a few pharmaceutical manufacturers using PAT as a key tool in the quality control process to many companies not yet engaging in the use of PAT at all. This article provides an overview on PAT, followed by an analysis for the use case of upgrading a container blending process with PAT. As such it is meant to be a guideline for management, technical operation and quality assurance in the understanding and decision towards the introduction of PAT.
Correspondence:
Dr. Hubertus Rehbaum, Dr. Rehbaum Technology Consulting, Friedrichstr. 68, D-10117 Berlin; e-mail: h.rehbaum@rehbaum.info
 | Dr. Hubertus Rehbaum Dr. Hubertus Rehbaum studierte Elektro- und Informationstechnik (Dipl.-Ing.) sowie Betriebswirtschaftslehre (Dipl.-Wirt.Ing.) an der RWTH Aachen. Während seiner Promotion in Angewandter Informatik an der Georg-August-Universität Göttingen entwickelte er Algorithmen für die Analyse von Biosignalen zur Steuerung von Handprothesen. Mit der Stelle als Manager Scientific Operations bei L.B. Bohle wechselte er anschließend in die Pharmaindustrie, um dort |
Schließen Sie hier ein Abonnement ab und profitieren Sie von den vielseitigen Nutzungsmöglichkeiten.